Inexpensive Multigram-Scale Synthesis of Cyclic Enamines and 3-*N* Spirocyclopropyl Systems
Type
Publication
Organic and Biomolecular Chemistry, 16(4), 652-656
Abstract
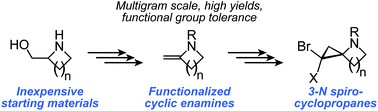
Cyclic enamines are important synthons for many synthetic and pharmacological targets. Here, we report an inexpensive, catalyst-free, multigram-scale synthesis for cyclic enamines with exocyclic double bonds and four- to seven-membered rings. This strategy is more conducive to scale up, permissive of functionalization around the cyclic system, and less sensitive to the nature of the N-protecting group than previously-described methods for cyclic enamine synthesis. Further, we explore application of these enamines to the synthesis of highly-strained spirocyclic 3N-cyclopropyl scaffolds.