Type
Publication
Organic Letters, 21(10), 3721-3725
Abstract
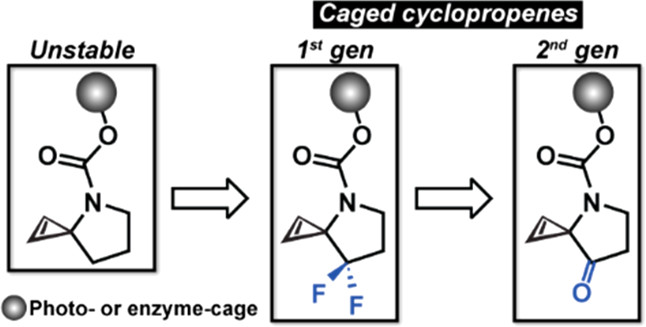
Activatable cyclopropenes are unreactive toward their inverse electron demand Diels-Alder reaction partner (e.g., s-tetrazines) until they are activated. The activation strategy is highly modular due to the cyclopropene’s ability to be caged by various light- and enzyme-activatable groups. This work describes the next generation of activatable cyclopropenes with a new core scaffold that maintains the activation modularity of the first generation but improves upon the ligation kinetics with s-tetrazines by ≤270-fold.