A Photocaged, Cyclopropene-Containing Analog of the Amino Acid Neurotransmitter Glutamate
Abstract
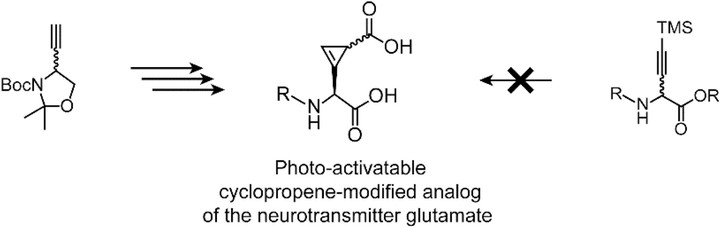
Substituted cyclopropenes serve as compact biorthogonal appendages that enable analysis of biomolecules in complex systems. Neurotransmitters, a chemically diverse group of biomolecules that control neuron excitation and inhibition, are not among the systems that have been studied using biorthogonal chemistry. Here we describe the synthesis of cyclopropene-containing analogs of the excitatory amino acid neurotransmitter glutamate starting from a Garner’s aldehyde-derived alkyne. The deprotected cyclopropene glutamate was stable in solution but decomposed upon concentration. Appending a light-cleavable group improved the stability of the cyclopropene while simultaneously caging the neurotransmitter. This strategy has the potential to permit deployment of cyclopropene-modified glutamate as a bioorthogonal probe of the neurotransmitter glutamate in vivo with spatiotemporal precision.